Did you know?
Here are some helpful tips and insights that will help you make informative decisions that result in better crops and yield.
Nutrient Interactions and Availability
In the soil, nutrients interact with one another leading to changes in availability to plants. The diagram below displays the various interactions that can occur.
Antagonism: High levels of a particular nutrient in the soil can interfere with the availability and uptake of other nutrients. For example, high nitrogen levels can reduce the availability of boron, potash, and copper; high phosphate levels can influence the uptake of iron, calcium, potash, copper, and zinc; high potash levels can reduce the availability of magnesium. Thus, the application of high levels of nitrogen, phosphorus, and potassium can induce plant deficiencies of other essential nutrients.
Stimulation: Occurs when high levels of a particular nutrient increase the plant’s demand for another nutrient. For example, increased nitrogen levels create a demand for more magnesium.
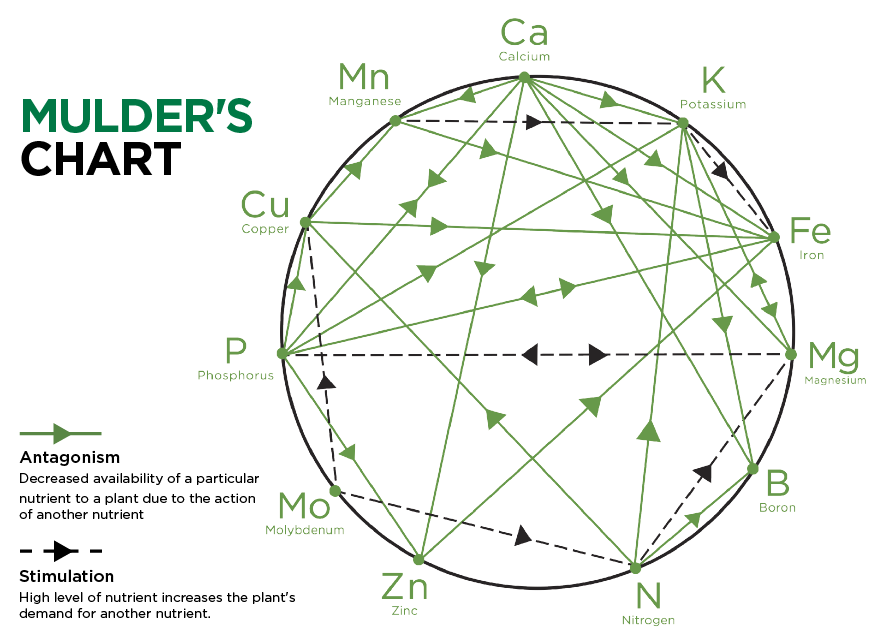
Mulder, D. (1953). Les elements mineurs en culture fruitiere. In Convegno Nazionale Frutticoltura (pp. 118–98). Montana de Saint Vincent.
Factors influencing penetration and absorption of foliar feeds
Temperature: Foliar sprays dry quickly when applied at high temperatures, reducing absorption of the nutrients in solution.
Light: High light intensities can improve foliar uptake.
Humidity: High humidity favors nutrient uptake through the leaves in two ways: by decreasing the rate of drying of the applied nutrient solutions, and by causing the cuticle to absorb water from the atmosphere and swell, which results in the formation of more polar pores.
pH of the Spray Solution: The pH of the spray solution can influence solubility, uptake, and penetration of the plant nutrients.
Leaf Age: Aging leaves develop thick cuticles that hinder foliar uptake, while young developing leaves have thin cuticles and are therefore more efficient at foliar uptake.
Uniform Application: Higher spray volumes result in more uniform coverage and more effective foliar feeding. Foliar applications should wet the entire canopy, especially the new leaves.
Concentration: As the concentration of the foliar spray increases, uptake also increases. However, lower spray water volumes generally result in less uniform spray coverage and less effective foliar feeding.
Wettability: Wetting agents improve foliar uptake by enhancing leaf coverage, leading to greater foliar uptake. Surfactants reduce the surface tension of the solution, overcoming cuticular barriers and improving foliar uptake.
Improving the uptake and mobility of foliar-applied nutrients
The cuticle provides plants with a layer of protection surrounding their stems, leaves, fruits, and flowers, which can be compared, in many ways, to our skin. The function of this cuticle is to control the flow of gases in and out of the plant and to maintain optimal levels of transpiration (the evaporation of water from the leaves). The primary obstacle to efficient foliar feeding is effective movement through the cuticle and into the plant tissue.
Two primary points of entry through the cuticle:
Stomata: Openings in the cuticle that open and close to control the exchange of gases and transpiration (water loss). Foliar applied nutrients can pass through the stomata and thus through the cuticle. However, this process is inefficient as stomata are primarily located on the underside of leaves. Also, stomata have the ability to open and close throughout the day and are frequently closed during optimal foliar spray times.
Polar Pores: Areas of water absorbing compounds in the cuticle that form pathways and allow certain nutrient sprays to diffuse through the cuticle and into the leaf. Many foliar fertilizers on the market are inefficient at being taken up by the leaves for a number of reasons. Foliar fertilizers must be soluble in order to readily move through the cuticle. Additionally, many fertilizers on the market (such as humates and lignosulfonates) are restricted from uptake via the polar pores due to their large molecular size. The efficient use of polar pores requires fully dissolved true solutions.

